Personalised diet improve intestine microbiotaand metabolism of obese rats
- ediensofficial
- 27 жовт.
- Читати 22 хв


Recent research on human microbiome provide opportunities to develop functional foods of new gen-
eration that can regulate intestinal microbiota and the biochemical status of the individual. The aim of the
study was to determine the effect of individually designed nutrition on the intestinal microbiota and metabolic
parameters of rats. Outbred laboratory rats with obesity were randomly divided into 9 groups (n = 12) de-
pending on the type of food ingredients taken orally for three months. The ratio of the intestinal commensal
microorganisms main groups, as well as the lipid profile and the content of glucose, urea, calcium in the
serum of animals were determined. It was shown that cholesterol level in the serum was reduced in experi-
mental groups after consumption of lactobacilli suspension, blueberry juice, fermented milk drink based on
lactobacilli, fermented milk drink with blueberry juice, sauerkraut. In most cases, the gut microbiome of ex-
perimental animals was characterized by a consistently high level of lacto and other beneficial bacteria and
decreased amount of opportunistic microorganisms at the end of the experiment compared with animals in
the control group. Based on the obtained data, we first proposed the principles of creating functional products
by synergistically combining components of edible plants that act as prebiotics and microorganisms that act
as probiotics for personalized use, targeted correction of intestinal microbiome and prevention of noncom-
municable diseases.
K e y w o r d s: functional foods, lipid profile, cholesterol, obesity, intestine microbiota, prebiotic and probio-tic components.
The fact that co-evolutionary relationships
between nutrition, gut microbiota, stress,
lifestyle, and environmental factors lead to
epigenetic influence on human health outcomes is
already widely accepted [1, 2]. Nowadays, it is ob-
vious that changes in gut microbiota often act as a
trigger of noncommunicable diseases connected to
low-grade inflammation and metabolic disorders,
such as obesity, type 2 diabetes (T2D), cardiovascu-
lar diseases, and others. Recently, clear evidence that
patients with such metabolic diseases face changes
in specific groups of gut microbiome has been re-
ported. In addition, most of the mechanisms of
commensal microorganisms’ modulation of human
health are being investigated [3-5]. For example, en-
teric carriage of a community of Clostridium species
induces IL-10 secreting Foxp3 Tregs in the colon,
probably via the induction of transforming growth
factor beta [6].
Recent scientific advances point to the fact that
low-grade inflammation is related to gut microbiota
dysfunction caused by microorganism’ imbalance
and contributing to obesity. This is currently one of
the most serious public health challenges worldwide
because of its increasing prevalence and its contribu-
tion to a complex of symptoms collectively called the
“metabolic syndrome” and other comorbidities, such
as type 2 diabetes [7, 8]. Diabetic individuals have lower counts of Bifidobacterium and Faecalibacte-
rium microbial representatives beneficial for gut [9].
The beneficial effect of the “healthy gut microbial
composition” and microorganisms’ ratio are recon-
sidered because of their different functional features
[10].
Nowadays, one of the commonly known and
most widely used approaches to correct human mi-
crobiota is to prescribe various biopharmaceuticals
like pre-, pro-, and synbiotics, or pharmabiotics,
if their efficacy is clinically proven. Among the
numerous gut microbial species, certain commen-
sal bacteria are known to provide health benefits to
the host when administered in adequate amounts,
and, as such, they are labeled “probiotics” [11]. In
addition to the reported limited success of biophar-
maceuticals’ use [12] in medical practice, which is
potentially increased through prescription personali-
zation [13], in our opinion, the most promising is still
a more integrated, “natural”, and targeted prognos-
tic correction of microbiota via the newly developed
functional nutrition of new generation [14].
One of the recognized beneficial mechanisms
of commensal gut microbiota effect on the host is
the production of specific short-chain fatty acids
(butyrate non acetate and propionate) during the fer-
mentation of dietary fibers. These acids have multi-
ple beneficial effects on the host’s energy metabo-
lism regulation on the whole: they not only improve
gut condition, but also directly affect all the other pe-
ripheral tissues of the host, including muscles, liver,
and nerves [15-17].
It has been proven that dietary microbiome ad-
justment prevents overweight and obesity. One of the
good examples is the “Mediterranean diet” based on
the use of bread, pasta, rice, corn porridge, cereals
and potatoes, fruits, vegetables, legumes, olive oil,
yogurt, and fish [18, 19]. However, data concern-
ing its efficacy still requires further confirmation by
properly arranged clinical trials [20].
Interestingly, individual members of the gut
microbiome can also have profound effects on
host mucosal homeostasis, and specific microbes
have been found to promote inflammatory [21, 22]
or anti-inflammatory [23, 24] responses in the gut
[25]. Hence, the interaction between the microbi-
ome and the gut immune system is crucial for the
maintenance of mucosal homeostasis. For example,
Bacteroides fragilis produces a polysaccharide A
which induces Tregs that secrete IL-10 and inhibit
gut inflammation [5, 26]. Additionally, B. fragilis
can produce a-galactosylceramide (a-Gal-CerBf), a
glycosphingolipid which is capable of binding CD1d
and activating invariant natural killer T cells [27].
Microbiota of every individual is unique and
has the function of modulating the immune system.
Therefore, individually designed diets that take into
account a person’s characteristics and are able to pre-
vent the development of infectious and diet-related
somatic pathologies can be considered promising.
Modern food is considered not only as com-
position of nutrients. It must also comply with in-
dividual requirements and have a positive effect at
the biochemical, cell, tissue, organ, and organism
levels. For example, Chardonnay grape seed flour
can modulate the gut microbiota while lowering ex-
cessive plasma cholesterol and improving the state
of the vascular wall [28]. Moreover, it is better to
choose food from a local source [29] so that it was
ethnic, tasty, and cheap. If we know the key micro-
organisms or their associations that are markers of a
certain disease, we may choose the food developed
to specifically modulate gut microbiota.
The aim of this study was to investigate the im-
pact of individually designed nutrition on gut micro-
biota and metabolism of rats.
Materials and Methods
Bacterial strains and animal groups. In the
study, we used our original strains sequenced and
documented in the Depositary of Microorganisms
of the D.K. Zabolotny Institute of Microbiology
and Virology of the NASU, namely: Lactobacillus
casei IMB B-7412 (isolated from sauerkraut), Lac-
tobacillus plantarum IMB B-7414 (isolated from
sauerkraut), Lactobacillus paracasei IMB B-7483
(isolated from Sautéed pickled green beans), and
L. plantarum KR-1 (isolated from Kvass southern).
Bacterial strains were obtained from fermented
products and were chosen based on their anti-inflam-
matory features, ability to specifically modulate lo-
cal immune response, and regulate gut microbiota
representatives, which was demonstrated in vitro
[30]. Pro- and anti-microbial activity of the pre- and
probiotic components of the complex novel foods
was investigated in vitro [31, 32].
All experiments in rats were performed in
accordance with the international principles out-
lined by the European Convention for the Protec-
tion of Vertebrate Animals Used for Experimental
and Other Scientific Purposes (Strasbourg, 1986)
signed by the Verkhovna Rada of Ukraine in 2002, Law of Ukraine No. 3447 - IV “On the Protection
of Animals from Cruelty”, meeting minutes of the
Bioethics Commission of the Medical Faculty of the
State University “Uzhhorod National University”
(Minutes No. 1, dated May 24, 2019).
In this study, nine groups of 12 white laborato-
ry rats aged 22-24 months, with male and female rats
being equally presented in each group, were formed.
For the experiments we have used rats additionally
fed with a “fat-rich” diet (in analogues to high-fat
diet rodent models [33]) for the induction of obesity.
We have specifically chosen the old rat generation
for the personalized diet experiments in order to ad-
dress “age” relevant issues and to be able to provoke
the “type 2 diabetes” like human condition connect-
ed with microbiome and metabolomic profile” that
are relevant to chronic inflammation changes and
which are often initiated in elderly people. In the
experimental groups, each animal received tested
ingredient(s) in the amount of 0.5 ml daily during 12
weeks in addition to the standard food received by
the control group. During the experiment, all ani-
mals were orally consuming different ingredient(s)
depending on the experimental group: Group 1 –
suspension of lactobacilli (L. casei IMB B-7412,
L. plantarum IMB B-7414, L. paracasei IMB
B-7483, and L. plantarum KR‐1); Group 2 – blueber-
ry juice (Vaccinium murtillus); Group 3 – fermented
milk drink with strains of lactobacilli (L. paracasei
IMB B-7483, L. casei IMB B-7412, L. plantarum
IMB B-7414, and L. plantarum KR-1) and blueberry
juice (the ratio of the fermented drink and blueberry
juice was 4:1); Group 4 – fermented milk drink with
strains of lactobacilli (L. paracasei IMB B-7483,
L. casei IMB B-7412, L. plantarum IMB B-7414,
and L. plantarum KR‐1) without plant components;
Group 5 – sauerkraut juice with L. casei IMB B-7412
and L. plantarum IMB B-7414; Group 6 – persimmon
juice (Diospyros kaki); Group 7 – lignin; Group 8 –
pectin (15%); Group 9 – control group, standard vi-
varium diet food. A suspension of microorganisms
was prepared daily before the administration using
48 h pure cultures of tested microorganisms taken at
the concentration of no less than 1.5×108
CFU/ml ac-
cording to McFarland. Body weight of each animal’s
group was determined twice, at the start and at the
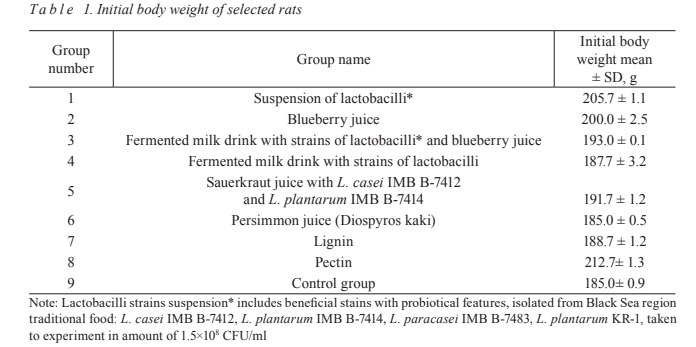
end of experiment (Table 1, Fig. 1).
Study of the biochemical and microbiological
parameters. Before and after the gavage, the weight
of the all the experimental animals was monitored
and blood sampling was taken from the tail vein
using the Microvette® capillary blood collection
system for biochemical study. The lipid profile,
namely total lipids, triglycerides, low-density lipo-
proteins (LDL), cholesterol, urea, calcium, and glu-
cose, was measured by colorimetric analysis using
ready-made reagents manufactured by “Philisit-
Diagnostics,” LLC.
In order to detect changes in major gut micro-
biota representatives and their composition under the
influence of tested ingredients of the newly develo-
ped functional nutrition in obese rats in real time,
the samples were taken on the third, seventh, 14th,
21st, 28th, 35th, 42nd, 49th, 56th, 63rd, 70th, 77th, 84th,
and 91st days from the beginning of the experiment.
Therefore, on every seventh day of the experiment
1 g of feces was collected from the experimental
animals and mixed with 1 ml of PBS. Ten-fold serial
dilution of samples was performed and plated cor-
respondingly on the chromogenic, typical, and se-
lective growth media: MacConkey agar, Blood agar,
Nutrient agar, Mannitol salt agar, Wilson-Blair agar,
Sabouraud dextrose agar, Lactobacillus MRS agar,
Bifidobacterium agar, Anaerobic blood agar, Bile
esculin agar, Streptococcus selective agar, Bacte-
roides bile esculin agar (all above mentioned growth
media produced by HiMedia Laboratories, India),
UriSelectTM 4 Medium (Bio-Rad Laboratories, Inc,
USA), or Blaurock semi-liquid modified hepatic me-
dium (Liofilchem, Italy).
For Bacteroides’ isolation, of 10-5–10-7 dilutions
of each sample, 10 μl were plated on the surface of
Bacteroides bile esculin agar and incubated for 4-5
days under anaerobic conditions. After microscopy,
the morphology of the microorganisms was evalua-
ted. Gram-negative polymorphic anaerobes can
be preassigned to Bacteroides. Lactobacilli were
isolated from 10-2–10-8 dilutions. Seeding was car-
ried out in the amount of 10 μl on MRS broth or
MRS agar and incubated for 2–3 days under anaero-
bic conditions.
Enterococci, staphylococci, and streptococci
were isolated by plating 10 μl of a 10-2–10-8 dilution
on bile esculin agar, mannitol salt agar, or strepto-
coccus selection agar respectively and incubated
at 37°C in a thermostat for 18-24 h. Blood agar,
nutrient agar, and UriSelect were also used for strep-
tococci isolation. Preliminary differential diagnosis
of Staphylococcus aureus was performed using tests
for catalase, hemolytic, and coagulase activity.
Yeast-like fungi were isolated by plating the
10‐2–10-8 dilution on the surface of sabouraud dex-
trose agar. After 2–3 days, white-matte colonies
were selected for identification. The total amount of
aerobic bacteria and their hemolytic properties was
determined by plating 10-5 and 10-7 suspension dilu-
tions on blood agar. The total amount of enterobac-
teria was determined by plating 10 μl of the suspen-
sion of 10-5–10-8 dilutions on MacConkey Agar.
Spore-forming bacteria, including clostridia,
were isolated by seeding 10 μl of 10-3–10-5 and 10-7
dilutions on Wilson-Blair agar. After 48 h of incuba-
tion, the number of black colonies in the agar depth
was calculated and formation of the gas separating
the nutrient medium was recorded.
Counting of all types of microorganisms was
carried out according to the formula: CFU/g = a×b×c,
where: a stands for the number of colonies grown on
the nutrient medium; b stands for dilution coefficient
dose (when plating 100 μl, a = 10; when seeding
50 μl, a = 5; when plating 10 μl, a = 100); and c
stands for dilution factor.
Identification of isolated microorganisms was
performed using biochemical test systems ANAE-
RO-23, ENTERO-24, NEFERM-test, Candida-23,
STAPHYtest 16, and STREPTOtest 24 (Erba La-
chema s.r.o., Czech Republic).
Statistical analysis. The experiments were per-
formed in triplicate. For the mathematical analysis of
the data, licensed software SPSS 17.0 was used. Par-
ametric results were reported as means with SD. The
normality of distribution was determined using the
Lilliefors criterion. In the group differences before
and after the intervention were evaluated using the
Wilcoxon rank-sum test. The differences between
the groups were evaluated using the Mann–Whitney
U test. P < 0.05 was considered significant. Correla-
tion relationships were determined using the Pearson
coefficient.
Results and Discussion
Under the influence of different ingredients –
potential components of the new generation func-
tional foods – body weight of the experimental ani-
mals significantly decreased (P < 0.05) in all groups
except the control one, where it grew by (20 ± 4) g,
and the fourth group of rats that followed the fer-
mented milk drink diet without plant components,
where we noted an increased body weight (P < 0.05)
by (15 ± 4) g (Fig. 1). LDL significantly decreased
(P < 0.05) under the influence of lactobacilli, 15%
apple pectin concentrate, lignins, and sauerkraut
juice, while in other experimental groups there were
no significant changes observed (P > 0.05) com-
pared to the control group where this parameter
significantly increased (P < 0.05), namely 2.5 times
(Fig. 2, B).
As a result of the introduction of the diets we
developed, total lipid content significantly decreased
(P < 0.001) in all animal groups except three groups:
one of them being the control group, where a signifi-
cant increase (P < 0.01) in the total lipid content was
noted, and in two experimental groups of animals
consuming apple pectin and lignin (Fig. 2, C). Cho-

lesterol reduction (P < 0.05) occurred in all experi-
mental groups of animals (Fig. 2, D). Interestingly,
only in case of apple pectin consumption triglyceride
concentration decreased (Fig. 2, A). A decrease in
the concentration of blood urea from (3.57 ± 0.03)
mmol/l to (0.9 ± 0.01) mmol/l (P < 0.01) was ob-
served only in the group of animals following a diet
enriched with probiotic bacterial strains (Fig. 3, A).
Glucose content decreased significantly (P < 0.01)
under the effect of blueberry juice and fermented
milk drink without plant components (Fig. 3, C),
but not when fermented milk drink with blueberry
extract was used separately. An increase in calcium
from (0.68 ± 0.03) mmol/l to (0.79 ± 0.05) mmol/l
(P < 0.01) was observed under the composition of
fermented milk drink without plant components.
When using all other diets, a decrease in calcium
levels was observed (Fig. 3, B).
Despite the increasing weight of animals in the
control group and animals consuming fermented
milk drink with lactobacilli without plant compo-
nents, these two groups significantly differed in other
lipid profile indices. In contrast to the control group,
animals consuming fermented milk drink without
plant components had an improvement in almost all
registered biochemical parameters (including a slight
decrease in total blood plasma lipids and a similar
increase in calcium levels).
In the study, we used elderly white rats with
obesity caused by age-related changes and feeding
them high-fat diet. Existing at the beginning of the
experiment and progressive metabolic disorders led

to an increase in the weight of animals in the con-
trol group in the absence of treatment and resulted
in a difference between the biochemical parameters
of the rats’ blood before and after the experiment.
The increase in weight of experimental animals of
group 4 is explained by the fact that long-term con-sumption of lactic acid products (on the example of our studied fermented beverage – fermented milk
product with selected LAB strains) leads to weight
gain due to their high fat and caloric content. There-
fore, when prescribing or selecting products, even a
healthy diet should be guided by their defined norms,

limited amount or fat content, and to ensure a balan-
ced diet, it is necessary to predict the effects of ex-
posure, which can be provided by our correlations of
prognostic corrections of the microbiome.
Our study demonstrated that animals can be
overweight not only because of increasing levels
of opportunistic microorganisms such as Staphylo-
coccus nepalensis and Enterococcus faecalis, but also because of the high level of the probiotic strain Bifidobacterium breve. Confirmed results of the
ability of blueberry juice (from 3.3 to 1.38 mmol/l)
and developed fermented milk drink (from 2.8 to
1.38 mmol/l) to regulate blood glucose levels in vivo
may have a prognostic value for recommending this
diet to patients with type 2 diabetes.
During the whole study we observed the fol-
lowing changes in rats’ gut microbiota under the in-
fluence of different diets. A consistently high level
of lactic acid and other beneficial bacteria and a de-
creasing level of opportunistic microorganisms were
detected in almost all experimental groups compared
to the control group.
Consumption of almost all of the developed
food components (diets) caused a decrease in the
concentration of E. faecalis and different Staphylo-
coccus species compared to that in the control group.
The level of Klebsiella pneumoniae and Morganella
morganii decreased significantly under the effect of
blueberry juice. The level of B. subtilis remained al-
most unchanged during the whole experiment under
the influence of the test samples based on plant ex-
tracts, plant pectins, and lignins.
Oral administration of lactobacillus strains sus-
pension (L. casei ІМВ В-7412, L. plantarum IMB
B-7414, L. paracasei IMB B-7483, and L. plan-
tarum KR-1) demonstrated an antagonistic activity
against Staphylococcus aureus, Peptostreptococcus
anaerobius, and E. faecalis and led to a decrease in
Escherichia coli and Enterobacter cloacae levels in
obese rats during the whole experiment (Fig. 4, A).
The concentration of commensal K. pneumo-
niae, M. morganii, E. coli, Actinomyces naeslundii,
and Bacteroides significantly decreased under the
influence of a blueberry juice-based diet. In this
study group, complete elimination of Streptococcus
parvulus and increase in E. faecalis and staphylo-
cocci were observed (Fig. 4, B).
Reduction in opportunistic microorganisms and
increase in beneficial microbiota indicated a positive
effect of consumption of the developed fermented
milk product with blueberry juice. Throughout the
study, we observed an increase in commensal lac-
tobacilli, elimination of Staphylococcus spp. and
E. faecalis, and a decreased concentration of E. coli
in this experimental group compared to the control
group (Fig. 5, A).
It was demonstrated that the 12-weeks oral ad-
ministration of fermented milk drink with strains of
lactobacilli (L. paracasei IMB B-7483, L. casei IMB
B-7412, L. plantarum IMB B-7414, and L. plantarum
KR-1) eliminated M. morganii and E. faecalis and
reduced E. coli and Actinomyces israelii concentra-
tions in obese rats (Fig. 5, B).
In this study, increased commensal lactoba-
cilli concentration, partial elimination of E. coli and
Bacillus subtilis, as well as complete elimination of
cocci were observed under the influence of sauer-
kraut juice (Fig. 6, A).
It should be noted that persimmon juice, in case
of long-term oral administration to animals, dem-
onstrated a significant antagonistic effect against
Staphylococcus cohnii and led to a decrease in the
levels of E. coli, Proteus mirabilis, E. cloacae, and
E. faecalis. In addition, consistently high levels of
microorganisms, such as Lactobacillus acidophilus,
Bifidobacterium longum, and B. subtilis ((5 ± 0.3)
×108CFU/g), were observed in case of persimmon
juice consumption compared to the control group
(Fig. 6, B).
As most vegetables and fruits contain dietary
fiber (pectin, lignin, cellulose, and hemicellulose),
which is a natural enterosorbent and can affect quan-
titative and qualitative composition of gut microbio-
ta, we investigated apple pectin and lignin effect in a
chronic experiment on obese rats.
The reduction in enterococci, B. subtilis,
E. coli, and P. mirabilis with a slight increase in
commensal lactobacilli concentration were observed
under the influence of lignin (Fig. 7, A).
Oral administration of pectin-based diet (apple-
pectin concentrate) demonstrated complete elimina-
tion of E. faecalis and a decrease in the concentra-
tion of commensal Bifidobacterium, staphylococci,
E. coli, and B. subtilis (Fig. 7, B).
Changes in gut microbiota of the control group
of animals demonstrated a decrease in the concen-
tration of E. faecalis and B. subtilis, as well as an
increase in the concentration of P. mirabilis (Fig. 8).
The reduction in Lactobacillus spp., B. subtilis,
and P. mirabilis in the gut microbiome may serve
as a biomarker of triglyceride increase and LDL
and cholesterol reduction (and vice versa). The in-
crease in B. breve and B. subtilis is a biomarker of
decreased plasma calcium (and vice versa).
In order to confirm the possibility of using
these microbial and biochemical parameters as bio-
markers, we searched for correlation between them
and classical disease signs, both at the beginning of
the study and after it.
In our study, such an indicator of lipid metabo-
lism as triglycerides closely correlates with LDL
(Pearson correlation coefficient is r = 0.7506) and
Lactobacillus spp. (r = 0.7130) in the gut microbiome
of rats. In turn, LDL inversely correlates with cho-
lesterol (r = -0.6772), while the inverse correlation of
cholesterol with the triglycerides (r = -0.7402) was
also observed. Overweight, as a baseline indicator

of abdominal fat distribution, directly correlates with
B. breve (r = 1,000) and S. nepalensis (r = 0.9244).
A correlation was found between B. breve and
E. faecalis (r = 0.9917) as well as between E. faecalis
and S. nepalensis (r = 1,000). In its turn, S. nepalen-
sis inversely correlates with B. breve (r = -0.5379).
Correlation was also found between P. mirabilis and
Lactobacillus spp. (r = 0.6776). In this study, the
change in the ratio of E. coli isolates with typical
and atypical enzymatic activity was investigated,
with the number of isolates with atypical enzymatic
activity predominating. It is interesting that the con-
centration of lactose-negative E. coli strains cor-
relates with the number of lactobacilli (r = 0.7608)
and Staphylococcus spp. (r = -0.7608) while Lacto-
bacillus reuteri correlates with Staphylococcus spp.
(r = 0.7456).
We also observed correlation between L. aci-
dophilus and S. aureus (r = 0.9979) in turn, S. au-
reus correlated with Staphylococcus epidermidis
(r = -0.8520), and S. epidermidis inversely correlated
with L. acidophilus (r = -1,000). A correlation be-

tween P. anaerobius and E. cloacae was established
(r = 0.6727).
In this study we detected correlation between
Bacteroides distasonis and M. morganii (r = 0.6811)
in turn, M. morganii correlated with K. pneumoniae
(r = 0.6811) and K. pneumoniae was negatively corre-
lated with B. distasonis (r = 1,000) while A. naeslun-
dii (r = 1,000) and A. naeslundii negatively correlated
with S. parvulus (r = -1,000). Negative correlation
of B. distasonis with S. parvulus (r = -1,000) and
S. parvulus with K. pneumoniae (r = 1,000) was also
established.
We found a direct correlation between the
amount of L. acidophilus and S. aureus in the in-
testinal contents of rats using the tested products.
Other scientists have studied that in obesity and ir-
ritable bowel syndrome observed the growth of op-
portunistic pathogens, including staphylococci and a
decrease in lactobacilli [34].
Also we can say that blood glucose is an am-
biguous indicator. Today, there is evidence that dif-
ferent strains of different LABs regulate blood glu-
cose levels differently - from no effect to a decrease
or increase in serum levels. In particular, according
to the European Journal of Clinical Nutrition [35],
men were given lactobacilli-based yogurt (L. acido-
philus) for three weeks and as a result the level of
HDL cholesterol, triglycerides and blood glucose in
the serum remained unchanged.
Another study shows that the strain of Lactoba-
cillus rhamnosus causes a decrease in glucose levels
[36]. There is a study according to which the level of
glucose depends on the content of calcium ions and
its reduction should not be interpreted unambiguous-

ly [37]. Therefore, to assess the effectiveness against
obesity, we first considered the expected (desired)
changes in lipid metabolism.
In fact, there are virtually no publications
linking biochemical and microbial indicators. These
parameters should be correlated with biochemical
and immune parameters. Our goal was a compre-
hensive study of all indicators to determine dynamic
changes. This direction was initiated by us and simi-
lar approaches were developed by us in the corre-
sponding articles [38].
The results of our study demonstrated positive
effects of separate components as well as the novel
functional food itself, namely fermented milk drink
with strains of lactobacilli and blueberry juice. Ap-
parently, this is due to the fact that probiotic strains
of lactobacilli and bifidobacteria create unfavorable
conditions for pathogens’ colonization by decreas-
ing their adhesive features. Additionally, probiotic
strains and plant extracts have the ability to adjust
and stimulate the beneficial gut microbiota of the ex-
perimental animals.

While analyzing the results of this study, it can
be argued that the suspension of lactobacilli L. para-
casei ІМВ В-7483, L. casei ІМВ В-7412, L. plan-
tarum ІМВ В-7414, and L. plantarum KR-1 demon-
strated the best hypocholesterolemic activity since
the long-term oral administration of suspension of
these strains led to a decrease in cholesterol levels
from 2.95 to 0.84 mmol/l.
As the glucose level in the serum of obese ani-
mals decreased due to oral administration of blue-
berry juice, as well as fermented milk drink with
strains of L. casei IMB B-7412, L. plantarum IMB
B-7414, L. paracasei IMB B-7483, and L. plantarum
KR-1, these particular components can be offered to
patients with type 2 diabetes.
It is interesting that the use of suspensions of
lactobacilli L. paracasei ІМВ В-7483, L. casei ІМВ
В-7412, L. plantarum ІМВ В-7414, and L. plantarum
KR-1, sauerkraut juice (enriched with strains of
L. plantarum IMB B-7414 and L. casei IMB B-7412),
15% apple pectin concentrate, and lignin cause a de-
crease in LDL levels. However, it should be noted
that a 15% apple pectin concentrate has the highest
hypolipidemic activity since the long-term oral ad-
ministration of this component leads to a decrease in
not only LDL, but also triglycerides and cholesterol.
That is why these components can be used to prevent
atherosclerosis and cardiovascular disease.

Conclusion. The results obtained during the
study, as well as the correlation between the changes
in key representatives of gut microbiota and values
of biochemical indicators of the organism’s state give
the possibility to develop the new generation func-
tional foods able to regulate gut microbiota balance
and prevent the development of diet-associated pa-
thologies.
Conflict of interest. Authors have completed
the Unified Conflicts of Interest form at http://ukr-
coi_disclosure.pdf and declare no conflict of interest.
Funding. This work is funded under the State
governmental budget of Ministry of Education and
Science, Topic: “Composite biological products from
microorganisms, plants and nanocompounds” Regis-
tration number 0113U002369.
Персоніфікована дієта
покращує мікробіоту
кишечника та метаболізм
щурів з ожирінням
В. В. Баті1, Т. В. Мелешко1
, О. В. Паллаг1,
І. П. Заячук2, Н. В. Бойко11
НДНЦ молекулярної мікробіології та
імунології слизових оболонок, Ужгородський
національний університет, Україна; 2
Кафедра фізіології та патофізіології, Ужгородський
національний університет, Україна;
e-mail: victoria.bati@uzhnu.edu.ua
Сучасні дослідження мікробіому людини
уможливлюють конструювання функціональ-
них продуктів харчування нового покоління,
здатних регулювати кишкову мікробіоту та
біохімічний статус індивідууму. Метою дослі-дження було з’ясувати вплив індивідуально роз-
робленого харчування на мікробіоту кишечника
та показники метаболізму в щурів. Безпородних
щурів із ожирінням було випадковим чином роз-
ділено на дев’ять груп (n = 12) залежно від типу
перорально вживаних протягом трьох місяців
інгредієнтів харчування. Визначали співвідно-
шення основних груп кишкових коменсальних
мікроорганізмів, а також ліпідний профіль та
вміст глюкози, сечовини, кальцію у сироватці
крові тварин. Встановлено зниження рівня хо-
лестеролу в сироватці крові дослідних тварин
після випоювання їм суспензії лактобактерій,
соку чорниці, кисломолочного напою на основі
лактобактерій, кисломолочного напою з соком
чорниці, квашеної капусти. У більшості випад-
ків кишковий мікробіом експериментальних
тварин характеризувався стабільно високим
рівнем лакто- та інших корисних бактерій та
зменшенням кількості умовно-патогенних мі-
кроорганізмів наприкінці експерименту порів-
няно з тваринами контрольної групи. На основі
одержаних даних нами вперше запропоновано
принципи створення функціональних продук-
тів нового покоління шляхом синергідного по-
єднання компонентів їстівних рослин (що діють,
як пребіотики) та мікроорганізмів (що діють, як
пробіотики) з метою їх прогностичного та пер-
соніфікованого застосування для попередження
виникнення некомунікативних захворювань,
шляхом спрямованої корекції мікробіому ки-
шечника макроорганізму, а відтак і його біохі-
мічного статусу.
К л ю ч о в і с л о в а: функціональні про-
дукти харчування, ліпідний профіль, холесте-
рол, ожиріння, мікробіота кишечника, пребіо-
тичні та пробіотичні компоненти.
References
1. D'Argenio V, Salvatore F. The role of the gut
microbiome in the healthy adult status. Clin
Chim Acta. 2015; 451(Pt A): 97-102.
2. Dickerson F, Severance E, Yolken R. The
microbiome, immunity, and schizophrenia and
bipolar disorder. Brain Behav Immun. 2017; 62:
46-52.
3. Ascher S, Reinhardt C. The gut microbiota:
An emerging risk factor for cardiovascular and
cerebrovascular disease. Eur J Immunol. 2018;
48(4): 564-575.
4. Virgin HW, Todd JA. Metagenomics and
personalized medicine. Cell. 2011; 147(1): 44-56.
5. Round JL, Mazmanian SK. Inducible Foxp3+
regulatory T-cell development by a commensal
bacterium of the intestinal microbiota. Proc Natl
Acad Sci USA. 2010; 107(27): 12204-12209.
6. Atarashi K, Tanoue T, Shima T, Imaoka A,
Kuwahara T, Momose Y, Cheng G, Yamasaki S,
Saito T, Ohba Y, Taniguchi T, Takeda K,
Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K.
Induction of colonic regulatory T cells by
indigenous Clostridium species. Science. 2011;
331(6015): 337-341.
7. Isolauri E, Rautava S, Collado MC, Salminen S.
Role of probiotics in reducing the risk of
gestational diabetes. Diabetes Obes Metab.
2015; 17(8): 713-719.
8. Sanz Y, Santacruz A, Gauffin P. Gut microbiota
in obesity and metabolic disorders. Proc Nutr
Soc. 2010; 69(3): 434-441.
9. Furet JP, Kong LC, Tap J, Poitou C, Basdevant A,
Bouillot JL, Mariat D, Corthier G, Doré J,
Henegar C, Rizkalla S, Clément K. Differential
adaptation of human gut microbiota to bariatric
surgery-induced weight loss: links with
metabolic and low-grade inflammation markers.
Diabetes. 2010; 59(12): 3049-3057.
10. Lloyd-Price J, Abu-Ali G, Huttenhower C. The
healthy human microbiome. Genome Med. 2016;
8(1): 51.
11. Sassone-Corsi M, Raffatellu M. No vacancy: how
beneficial microbes cooperate with immunity to
provide colonization resistance to pathogens. J
Immunol. 2015; 194(9): 4081-4087.
12. Pat. No 90789 A Technique for the prevention
of purulent complications and wound infection
after tooth extraction with bacterial suspension
based on Bacillus subtilis 090 / Petrov VO,
Rusyn VI, Boiko NV. Publ. 10.06.2014 Bul.
No 11. (In Ukrainian).
13. Pat. No 93301. Composite biological product
for the treatment of inflammation of
periodontal tissues and correction of associated
gastroduodenal disorders of the intestine in
children / Melnyk VS, Diachuk EY, Bati VV,
Levchuk OB, Boiko NV. Publ. 25.09.2014, Bul.
No 18. (In Ukrainian).
14. Ercolini D, Fogliano V. Food Design To Feed the
Human Gut Microbiota. J Agric Food Chem.
2018; 66(15): 3754-3758.
91
15. Kasubuchi M, Hasegawa S, Hiramatsu T,
Ichimura A, Kimura I. Dietary gut microbial
metabolites, short-chain fatty acids, and host
metabolic regulation. Nutrients. 2015; 7(4):
2839-2849.
16. Donohoe DR, Garge N, Zhang X, Sun W,
O'Connell TM, Bunger MK, Bultman SJ. The
microbiome and butyrate regulate energy
metabolism and autophagy in the mammalian
colon. Cell Metab. 2011; 13(5): 517-526.
17. Desbonnet L, Clarke G, Traplin A, O'Sullivan O,
Crispie F, Moloney RD, Cotter PD, Dinan TG,
Cryan JF. Gut microbiota depletion from early
adolescence in mice: Implications for brain and
behaviour. Brain Behav Immun. 2015; 48: 165-
173.
18. Enck P, Zimmermann K, Rusch K, Schwiertz A,
Klosterhalfen S, Frick JS. The effects of
maturation on the colonic microflora in infancy
and childhood. Gastroenterol Res Pract. 2009;
2009: 752401.
19. Trichopoulou A, Naska A, Orfanos P,
Trichopoulos D. Mediterranean diet in relation
to body mass index and waist-to-hip ratio: the
Greek European Prospective Investigation into
Cancer and Nutrition Study. Am J Clin Nutr.
2005; 82(5): 935-940.
20. Garcia-Mantrana I, Selma-Royo M, Alcantara C,
Collado MC. Shifts on Gut Microbiota
Associated to Mediterranean Diet Adherence
and Specific Dietary Intakes on General Adult
Population. Front Microbiol. 2018; 9: 890.
21. Ivanov II, Atarashi K, Manel N, Brodie EL,
Shima T, Karaoz U, Wei D, Goldfarb KC,
Santee CA, Lynch SV, Tanoue T, Imaoka A,
Itoh K, Takeda K, Umesaki Y, Honda K,
Littman DR. Induction of intestinal Th17 cells
by segmented filamentous bacteria. Cell. 2009;
139(3): 485-498.
22. Ivanov II, Frutos RdeL, Manel N, Yoshinaga K,
Rifkin DB, Sartor RB, Finlay BB, Littman DR.
Specific microbiota direct the differentiation of
IL-17-producing T-helper cells in the mucosa
of the small intestine. Cell Host Microbe. 2008;
4(4): 337-349.
23. Mazmanian SK, Round JL, Kasper DL. A
microbial symbiosis factor prevents intestinal
inflammatory disease. Nature. 2008; 453(7195):
620-625.
24. Round JL, Mazmanian SK. Inducible Foxp3+
regulatory T-cell development by a commensal
bacterium of the intestinal microbiota. Proc Natl
Acad Sci USA. 2010; 107(27): 12204-12209.
25. McDermott AJ, Huffnagle GB. The microbiome
and regulation of mucosal immunity.
Immunology. 2014; 142(1): 24-31.
26. Mazmanian SK, Round JL, Kasper DL. A
microbial symbiosis factor prevents intestinal
inflammatory disease. Nature. 2008; 453(7195):
620-625.
27. Wieland Brown LC, Penaranda C,
Kashyap PC, Williams BB, Clardy J,
Kronenberg M, Sonnenburg JL, Comstock LE,
Bluestone JA, Fischbach MA. Production of
α-galactosylceramide by a prominent member
of the human gut microbiota. PLoS Biol. 2013;
11(7): e1001610.
28. Kim H, Kim DH, Seo KH, Chon JW, Nah SY,
Bartley GE, Arvik T, Lipson R, Yokoyama W.
Modulation of the intestinal microbiota is
associated with lower plasma cholesterol and
weight gain in hamsters fed chardonnay grape
seed flour. J Agric Food Chem. 2015; 63(5):
1460-1467.
29. Deller S, Canto A, Brown L. Food access, local
foods, and community health. Community Dev.
2017; 48(5): 657-680.
30. Bati VV, Boyko NV. The biological properties
of lactоbacilli strains isolаted from food of plant
origin and edible plants. ScienceRise. 2016;
8(1(25)): 6-14. (In Ukrainian).
31. Bati VV, Boyko NV. Microbiological analysis
of sauerkraut in the process of its fermentation
according to the traditional and modernized
technologies. Microbiol Biotechnol. 2017;
(2(38)): 90-100.
32. Bati VV, Boyko NV. Novel functional food for
prevention of non-communicable diseases. Biol
Stud. 2019; 13(1): 71-84.
33. Buettner R, Parhofer KG, Woenckhaus M,
Wrede CE, Kunz-Schughart LA, Schölmerich J,
Bollheimer LC. Defining high-fat-diet rat
models: metabolic and molecular effects of
different fat types. J Mol Endocrinol. 2006;
36(3): 485-501.
34. Grygoruk GV, Myshchuk VG, Tserpiak NV.
Changes in gut microbiota and blood lipid profile
in patients with irritable bowel syndrome in
association with obesity. Bukovin Med Herald.
2019; 23(1(89)): 32-38. (In Ukrainian).
35. Schaafsma G, Meuling WJ, van Dokkum W,
Bouley C. Effects of a milk product, fermented
by Lactobacillus acidophilus and with fructo-
V. V. Bati, T. V. Meleshko, O.V. Pallah et al.
92
ISSN 2409-4943. Ukr. Biochem. J., 2021, Vol. 93, N 4
oligosaccharides added, on blood lipids in male
volunteers. Eur J Clin Nutr. 1998; 52(6): 436-
440.
36. Farida E, Nuraida L, Giriwono PE, Jenie BSL.
Lactobacillus rhamnosus Reduces Blood
Glucose Level through Downregulation
of Gluconeogenesis Gene Expression in
Streptozotocin-Induced Diabetic Rats. Int J
Food Sci. 2020; 2020: 6108575.
37. Bartlett PJ, Gaspers LD, Pierobon N, Thomas AP.
Calcium-dependent regulation of glucose
homeostasis in the liver. Cell Calcium. 2014;
55(6): 306-316.
38. Golubnitschaja O, Topolcan O, Kucera R,
Costigliola V, EPMA. 10th Anniversary of the
European Association for Predictive, Preventive
and Personalised (3P) Medicine - EPMA World
Congress Supplement 2020. EPMA J. 2020;
11(Suppl 1): 1-133.


Коментарі