EDIBLE FRUITS EXTRACTS AFFECT INTESTINALMICROBIOTA ISOLATED FROM PATIENTS WITHNONCOMMUNICABLE DISEASES ASSOCIATEDWITH CHRONIC INFLAMMATION
- ediensofficial
- 27 жовт.
- Читати 14 хв

The aim of our study was to investigate the gut microbiota in patients with noncommunicable diseases associated with chronic inflammation, namely obesity, type 2 diabetes, atherosclerosis, and cardiovascular disease as well as to find out potential ability of edible plants fruits extracts to inhibit the
growth of selected conditionally pathogenic microorganisms.
Limited clinical trial was performed and gut microbiota analysis was done using routine methods and qPCR. The antibacterial properties of edible plants fruits in relation to the selected potentially patho genic microorganisms were studied.
The composition of the intestinal microbiota of obese patients was characterized by an increase in thenumber of Enterococcus spp. and Lactobacillus spp. along with a decrease in the amount of Escherichiacoli. Decreases in E. coli and lactobacilli were observed in patients with type 2 diabetes. In atherosclerosis,an increase in streptococci, enterococci, and enterobacteria was observed, whereas in patients with car-diovascular disease there was an additional increase in staphylococci and candida along with a decrease in E. coli. Decreases in Bifidobacterium spp., Bacteroides spp., Roseburia intestinalis and Akkermansia muciniphila were observed in patients of all groups. The growth of Klebsiella spp. was inhibited by red currant and plum extracts; Enterobacter spp. — sweet cherry extract; Proteus spp. — extracts of blue-berry and cornelian cherry; Staphylococcus spp. — the extracts of black currant, sweet cherry, plum, jostaberry, alycha and cornelian cherry.
The obtained data can be used for early diagnosis of noncommunicable diseases and for their prevention with the help of personalized nutrition.
According to the World Health
Organization (WHO), non-communicable
diseases (NCDs) are chronic diseases that
are not transmitted from person to person,
have a long course, and progress slowly. In
the late twentieth century, NCDs turned
into a global epidemic and one of the greatest
threats to human life and health. According
to the WHO, 40 million people die annually
from NCDs, which accounts for 70% of all
deaths in the world [1]. NCDs result from a
combined influence of genetic, physiological,
environmental, and behavioral factors [2].
Studies of changes in the intestinal
microbiome and its role in the occurrence
of NCDs have become extremely relevant in
recent years [3]. Microbiome is part of human
physiology and is significantly involved in a
wide range of vital physiological processes,
including energy homeostasis and metabolism,
synthesis of vitamins and other important
nutrients, endocrine signaling, prevention
of colonization by pathogens, regulation
of immune function, and metabolism of
xenobiotics, carcinogens, and other harmful
compounds [4].
Persistent low-grade inflammatory
response underscores metabolic syndrome and
is a risk factor for cardiovascular diseases
(CVDs) [5, 6]. Inflammatory markers are
associated with obesity and the risk of obesity-
related CVDs [7]. Perturbation of intestinal
microbiota and changes in gut permeability
are triggers for the chronic inflammatory
state [8]. “Metabolic endotoxaemia” is a term
used to describe a link between gut bacteria,
endotoxins, and their circulating levels, with
inflammatory-induced obesity and metabolic
diseases linking it to CVDs [9].
Some research studies [10] demonstrated
that intestinal microbiota changes related to
obesity lead to threshold inflammation. In
obese people, intestinal microbiota changes
stimulate the absorption of monosaccharides
due to the increased number of capillaries
in the small intestine epithelium [11] and
significantly increase the ability to obtain
more energy from food by increasing the
number of microorganisms capable of
fermenting indigestible carbohydrates in the
colon [12, 13]. Obesity is a major risk factor
for the development of type 2 diabetes (T2D),
which leads to the destruction of insulin
receptors and causes resistance to insulin. In
turn, patients with diabetes also tend to suffer
from comorbidities, such as hypertension and
dyslipidemia, which further accelerates the
atherosclerotic process, and, therefore, such
patients have an extremely high cardiovascular
risk [14]. Atherosclerosis is a major risk
factor for CVDs assuming accumulation of
cholesterol and macrophages on arteries
walls, thus contributing to the formation
of atherosclerotic plaques [15]. Recent
studies suggest that intestinal microbiota
disruption may also enhance development of
atherosclerosis and CVDs [16, 17].
In our opinion, among numerous NCDs, it
is necessary to distinguish a group of diseases
directly relating to changes in the microbiome
and the main trigger of which is chronic
inflammation, that is obesity, T2D mellitus,
atherosclerosis, and CVDs.
Nutrition is the most important factor
that regulates gut microbiota composition.
Personalized nutrition is one of the most
effective approaches for prevention and
treatment of NCDs [18]. The edible plants’
fruits which are characterized by high
biologically active compounds (BAC)
contents and ability to stimulate the growth
of beneficial microorganisms and inhibit
the growth of conditionally pathogenic
microorganisms could be perspective
components for personalized nutrition.
Therefore, the aim of our research was to
study intestinal microbiota in patients with
NCDs related to chronic inflammation, namely
obesity, T2D mellitus, atherosclerosis, and
CVDs as well as to find out potential ability
of edible plants fruits extracts to inhibit the
growth of selected conditionally pathogenic
microorganisms.
Materials and Methods
Participants and study design
In order to study gut microbiota in patients
with NCDs related to chronic inflammation,
we performed a limited clinical case study, in
which four groups were formed: 1 — patients
with obesity; 2 — patients with type 2 diabetes;
3 — patients with atherosclerosis; 4 — patients
with cardiovascular diseases. In order to
achieve this goal, we examined 10 people from
each group.
The inclusive criteria for obesity were the
value of the body mass index (BMI), which
exceeds () 30 kg/m2 [19]; signed informed
consent to participate in the study. Exclusion
criteria: smoking, alcohol or drug use, diabetes
mellitus, CVDs, clinically significant kidney
or liver disease (or other organs and organ
systems), acute inflammatory diseases at
the time of examination, cancer; significant
lifestyle changes, mainly of dietary habits and
physical activity in the period shorter than 6
months.
Patients with T2D were selected according
to the criteria typical of this nosology [20]:
fasting plasma glucose 6.1 mmol/l; impaired
glucose tolerance — two hours after the oral
dose a plasma glucose 7.8–11.1 mmol/l;
glycated hemoglobin (HbA1c) 6.5%; signed
informed consent to participate in the study.
Exclusionary criteria were smoking, alcohol,
or drug abuse; pregnancy; an unstable medical
status; significant lifestyle changes, mainly
of dietary habits and physical activity in the
period shorter than 6 months. No participants
had clinically significant cardiovascular, renal
or liver disease or a history of cancer.
Inclusion criteria for atherosclerosis were
[21]: patients with a BMI in the range of normal
weight; low cardiovascular risk (SCORE 1%);
total cholesterol level below 8 mmol/l; total triglycerides levels below 2.3 mmol/l; signed
informed consent to participate in the study.
Exclusion criteria: patients receiving lipid-
lowering therapy (statins, ezetimibe, etc.) or
patients who do not meet the minimum period
of 3 months of discontinuation of therapy;
the lipid profile outside the inclusion criteria;
diabetes mellitus; SCORE > 1%; proven
secondary causes of dyslipidemia; presence of
manifest cardiovascular system disease in the
form of coronary artery disease, past stroke,
TIA, MI, etc.; presence of acute diseases,
chronic deterioration, or presence of infection,
which may distort the laboratory parameters;
significant lifestyle changes, mainly of dietary
habits and physical activity in the period
shorter than 6 months.
The following inclusion criteria were
used to select patients with CVDs: diagnosed
coronary heart disease, stroke, carotid artery
stenosis [22]; SCORE 5%; hyperlipidemia;
signed informed consent to participate in
the study. Exclusion criteria were smoking,
alcohol, or drug abuse; pregnancy; an unstable
medical status; clinically significant renal or
liver disease, acute inflammatory diseases at
the time of examination or a history of cancer;
significant lifestyle changes, mainly of dietary
habits and physical activity in the period
shorter than 6 months.
The Transcarpathian Regional Clinical
Cardiology Dispensary was the place of
inpatient examination of patients diagnosed
with atherosclerosis and CVDs, and for
patients with obesity and T2D — the
therapeutic department of the Mukachevo
Central District Hospital.
According to the conclusions of the
Commission on Biomedical Ethics (Protocol
No6/1 of 26.05.2020), all studies were
performed in compliance with the basic
provisions of the Good Clinical Practice
(GMP) (1996), Convention on Human Rights
and Biomedicine of the Council of Europe
(04.04.1997), the World Medical Association
Declaration of Helsinki — Ethical Principles
for Medical Research Involving Human
Subjects (1964–2013), and the orders of
the Ministry of Health of Ukraine No690 of
23.09.2009 and No616 of 03.08.2012, in which
a person is an object of research. All patients
gave informed consent to participate in the
study.
Analysis of gut microbiota
In order to study gut microbiota the
faecal samples were diluted with pre-reduced
phosphate-buffered saline (PBS), then the ten-
fold serial dilution of samples was performed
in PBS and plated correspondingly on the
following nutrient media: Mitis Salivarius
Agar, Bile Esculin Agar, Mannitol Salt
Agar, Endo Agar, Bismuth Sulphite Agar,
HiCrome Clostridial Agar, Sabouraud
Dextrose Agar, Lactobacillus MRS Agar,
Bifidobacterium Agar, Bacteroides bile
esculin agar, Propionibacter Isolation Agar,
L.D. Esculin HiVegTM Agar (manufactured
by HiMedia Laboratories, India), UriSelectTM
4 Medium (Bio-Rad Laboratories, Inc, USA),
and Blaurock semi-liquid modified hepatic
medium (manufactured by Liofilchem, Italy).
Identification of isolated microorganisms was
performed using biochemical test systems
ANAERO-23, ENTERO-24, NEFERM-test,
Candida-23, STAPHY-16, and STREPTO test
24 (Erba Lachema s.r.o., Czech Republic).
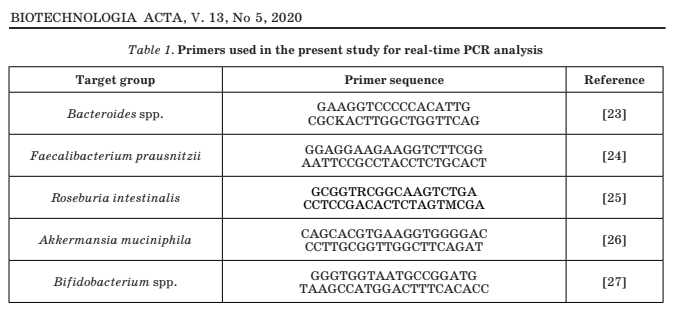
Real-time polymerase chain reaction
(qPCR) was performed on an AriaMx
instrument (manufactured by Agilent
Technologies, USA) using specific primers
(Table 1). Isolation of bacterial DNA was
performed using the ZymoBIOMICS DNA
Mini Kit (Zymo Research, USA) according to
the instructions for use. The concentration of
isolated DNA in the samples was checked on
a DeNovix DS-11 FX + spectrophotometer/
fluorometer (DeNovix Inc., USA).
Extracts preparation
Using GrindomixTM electric mixer,
we obtained native homogenates of the
following edible plants’ fruits (grown in the
mountainous regions of Zakarpattia): Ribes
rubrum (red currant), Prunus avium (sweet
cherry), Prunus x domestica (plum), Ribes x
nidigrolaria (jostaberry), Vaccinium myrtillus
(blueberry), Ribes nigrum (black currant),
Prunus cerasifera (alycha) and C rnus mas L.
(cornelian cherry). The obtained homogenates
were filtered through nylon nanofilters with a
pore width of 44 μm (BD Falcon, USA).
We studied the antibacterial properties of
the above-mentioned edible plants fruits in
relation to the selected microorganisms such
as Escherichia coli, Enterobacter cloacae,
Klebsiella pneumoniae, Klebsiella oxytoca,
Proteus mirabilis, Pseudomonas aeruginosa,
Streptococcus pyogenes, Staphylococcus
aureus, Enterococcus faecalis, Candida
albicans by culturing them in extracts obtained
from these edible plants’ fruits [23]. The initial
concentration of the selected bacterial strains
was 1.5108 CFU/ml. After 24, 48 and 72
hour of their co-incubation the ten-fold serial
dilution of samples was performed and plated
correspondingly on an appropriate nutrient
medium. The test cultures of microorganisms
without edible plants fruits extracts were the
control in the study.
Data analysis
Statistical analyses were performed using
the statistical program Origin 2019 (OriginLab
Corporation, USA). All data are presented as
median and interquartile range or the mean
± SD. Nonparametric comparisons were done
using multiple comparisons Kruskal-Wallis
ANOVA with Dunn’s Test as post-hoc analysis.
P values < 0.05 were considered statistically
significant. Normally disturbed date were
compared using student’s t-test.
Results and Discussion
Among the coccal microorganism forms
of the intestinal microbiota of obese patients,
there was a significant increase in the
amount of enterococci, while the amounts of
streptococci and staphylococci were within
the norm. The level of bacteria of the genera
Enterococcus spp., Streptococcus spp., and
Staphylococcus spp. in the gut microbiota
of patients with T2D was within the norm.
In patients with atherosclerosis, intestinal
microbiota demonstrated an increase in the
amounts of enterococci and streptococci
along with the normal value of staphylococci
amount. Under CVDs, patients showed an
increase in the amounts of bacteria of the
genera Enterococcus spp., Streptococcus spp.,
and Staphylococcus spp. in gut microbiota
(Fig. 1).
Analysis of the obtained data reveals
that an significant increase in the amount of
staphylococci within intestinal microbiota was
characteristic only of the group of patients
with CVDs, while an increase in the amount
of streptococci was observed in patients with
atherosclerosis and CVDs. An increase in the
amount of enterococci was observed in patients
with obesity, atherosclerosis, and CVDs.
Therefore, an increase in the amount of coccal
microorganism forms, namely Enterococcus
spp., Streptococcus spp., and Staphylococcus
spp., within gut microbiota may indicate the
development of atherosclerosis and CVDs.
In the intestinal microbiota of patients
with obesity and T2D, Enterobacteriaceae
demonstrated a significant decrease in the
amount of normally fermenting Escherichia
coli at the normal concentration of Proteus
vulgaris, Klebsiella spp., and Enterobacter
spp. In patients with atherosclerosis, gut
microbiota demonstrated a significant
increase in the amount of Klebsiella spp. and
Enterobacter spp., while the amounts of E. coli
and P. vulgaris slightly exceeded the norm.
In the intestinal microbiota of patients with
CVDs, there was an increase in the amounts of
Enterobacter spp. and P. vulgaris, a decrease
in the value of E. coli, and a normal amount of
Klebsiella spp. (Fig. 2).
According to the data obtained in the
study, an increase in the amount of Klebsiella
spp. was characteristic only of patients with
atherosclerosis, while an increase in the
amounts of P. vulgaris and Enterobacter spp.
was observed in patients with atherosclerosis
and CVDs. The concentration of E. coli was
below the norm in patients with obesity, T2D,
and CVDs, but in patients with atherosclerosis
there was a slight excess of this bacterium.
Given the above, an increase in the amount of
enterobacteria, especially Klebsiella spp. and
Enterobacter spp., indicates the development
of atherosclerosis.


Anaerobic and facultative-anaerobic gut
microbiota of obese patients was characterized
by an increase in the value of Lactobacillus
spp., normal values of Clostridium spp.,
Faecalibacterium prausnitzii and yeast-
like fungi of the genus Candida, as well as a
decrease in the levels of Bifidobacterium spp.,
Bacteroides spp., Roseburia intestinalis, and
Аkkermansia muciniphila.
In the intestinal microbiota of patients
with T2D, there was a decrease in the amounts
of Bifidobacterium spp., Lactobacillus spp.,
Bacteroides spp., F. prausnitzii, R. intestinalis,
and A. muciniphila, while the amounts of
yeasts of the genus Candida and Clostridium
spp. were within the norm. In the intestinal
microbiota of patients with atherosclerosis
and CVDs, there was a decrease in the
values of Bifidobacterium spp., Bacteroides
spp., F. prausnitzii, R. intestinalis, and
A. muciniphila, as well as normal values
of Lactobacillus spp., Clostridium spp.
An significant increase in the amount of
Candida spp. within intestinal microbiota was
characteristic only of the group of patients
with CVDs (Fig. 3 and Fig. 4).
While analyzing the data obtained, we could
see that an increase in the concentration of
lactobacilli within gut microbiota is observed in
obese patients, a decrease in the concentration
of Lactobacillus spp. is characteristic of
the microbiota of patients with T2D, while
in patients with atherosclerosis and CVDs
the amount of lactobacilli is within the
norm. Intestinal microbiota of patients
of all groups was characterized by normal
amounts of Clostridium spp., while the normal
concentration of F. prausnitzii was observed
only in patients with obesity. Decrease in the
amounts of Bifidobacterium spp., Bacteroides
spp., R. intestinalis, and A. muciniphila within
gut microbiota was observed in patients of all
nosological groups.
In previous studies, we obtained data
demonstrating the content of biologically
active compounds of selected edible plants
fruits and their potential ability to stimulate
the growth of lactic acid bacteria [29]. Here
we present the results of the studied effect of
edible plants fruits extracts on commensal,
beneficial, potentially pathogenic bacterial
strains (Table 2).
According to the data obtained (Table 2), the
red currant extract totally inhibited the growth
of K. pneumoniae, K. oxytoca, and P. aeruginosa
on 48 h of co-cultivation as well as S. aureus
on 72 h of co-cultivation. The extract of black
currant totally inhibited growth of S. aureus
after 48 h of co-cultivation and K. pneumoniae,
K. oxytoca, and P. aeruginosa on 72 h of co-
cultivation. The sweet cherry extract totally
inhibited growth of E. cloacae and S. aureus on
48 h of co-cultivation as well as K. pneumoniae,
K. oxytoca, P. aeruginosa, E. faecalis, and P.
mirabilis on 72 h of co-cultivation. The growth
of such bacterial strains as K. pneumoniae,
K. oxytoca, S. aureus, and C. albicans was
totally inhibited by plum extract on 48 h of
co-cultivation, while the strains of E. coli, E.
cloacae, P. mirabilis, P. aeruginosa, and S.
pyogenes strains were totally inhibited after
72 h of co-cultivation. The jostaberry extract
totally inhibited growth of P. aeruginosa
and S. aureus after 48 h of co-cultivation and
E. cloacae after 72 h of co-cultivation. The
extract of blueberry on 48 h of co-cultivation
totally inhibited growth of P. mirabilis as well
as s K. pneumoniae, K. oxytoca, and S. aureus
after 72 h of co-cultivation. The growth of P.
aeruginosa and S. aureus was totally inhibited
by alycha extract on 48h of co-cultivation,
while such bacterial strains as E. cloacae,
K. pneumoniae, K. oxytoca, P. mirabilis,
E. faecalis, and C. albicans totally inhibited
after 72 h of co-cultivation. The cornelian
cherry extract totally inhibited growth of P.
mirabilis, S. aureus, and C. albicans after 48 h
of co-cultivation.
Analyzing the data obtained in these
study, it can be concluded that extracts
of red currant and plum can be used for
inhibition of the growth of Klebsiella spp.;
the extract of sweet cherry can be used for
inhibition of Enterobacter spp. growth; the
extracts of blueberry and cornelian cherry
are effective growth inhibitors of Proteus
spp.; the extracts of plum and cornelian
cherry can be used for growth inhibition of
Candida spp.; the extracts of black currant,
sweet cherry, plum, jostaberry, alycha
and cornelian cherry are effective growth
inhibitors of Staphylococcus spp.
Current research studies consider
a potential role of gut microbiota in
the development of obesity and related
comorbidities. Gut microbiota can influence
energy extraction from food, lipid metabolism,
immune response, and endocrine functions
and its profile has shown differences between
obese and non-obese subjects [30]. Our study
revealed that intestinal microbiota of obese
patients was characterized by a sharp increase
in the amount of enterococci and a decrease in
the amounts of normally fermenting E. coli
and bifidobacteria, which are early diagnostic
markers of metabolic disorders [31].





A close relationship between the
gut microbe-dependent production of
trimethylamine-N-oxide (TMAO), derived
from specific dietary nutrients, such
as choline and carnitine, and future
cardiovascular events has been widely
recognized [32]. Trimethylamine (TMA),
which is produced by gut microbial enzymes
TMA lyases, is a precursor of TMAO. As
different gut microbial compositions generate
different levels of TMAO [33], higher blood
TMAO levels and an increased development
of atherosclerosis and CVDs risk can be
attributed to a TMA-producing microbiome harboring TMA lyases. Our research results
demonstrate that intestinal microbiota of
patients with atherosclerosis and CVDs is
characterized by an increase in the amounts
of Streptococcus spp., E. coli, and Klebsiella
spp. This is confirmed by the fact that these
bacteria are able to produce TMA [34].
One of the most important metabolic
activity of gut microbiota is the production
of non-gaseous SCFAs (acetate, propionate,
and butyrate), through fermentation of
microbiotaaccessible, complex carbohydrates
(e.g., oligosaccharides, resistant starch,
and plant cell wall materials) [35]. Butyrate
plays a significant role in the maintenance
of intestinal epithelial cell integrity with
important functions in the prevention
of ‘leaky gut’ associated with diabetes.
Therefore, the role of SCFA, particularly
butyrate and butyrate-producing bacteria
such as Bifidobacterium spp., Bacteroides
spp., F. prausnitzii and R. intestinalis are
crucial for health in obesity and diabetes [36].
Taking into account this fact we can conclude
that the decreased level of butyrate-producing
bacteria indicates inflammation processes
which are associated with NCDs.
Thus, from the work presented here, it
can be concluded that the gut microbiota
alteration contributes to the development
of NCDs such as obesity, T2D mellitus,
atherosclerosis, and CVDs. Thus, such
knowledge can be applied in early
diagnosis of those diseases. Analyzing the
experimental data obtained, and taking
into account results of our previous
studies, we can suggest that selected
edible plants fruits extracts can be used as
components of personalized nutrition for
prevention and treatment of NCDs related
to chronic inflammation. However, in vivo
investigations are necessary to confirm the
interactions between microbiota modulating
and intestinal beneficial effects.
This work was supported by the Ministry
of Education and Science of Ukraine, grant
no. 0117U000379 the introduction of new
approaches to the creation and use of modern
pharmabiotics.
ЕКСТРАКТИ ЇСТІВНИХ ПЛОДІВ
ВПЛИВАЮТЬ НА КИШКОВУ
МІКРОБІОТУ, ІЗОЛЬОВАНУ
У ПАЦІЄНТІВ З НЕКОМУНІКАТИВНИМИ
ЗАХВОРЮВАННЯМИ, ПОВ’ЯЗАНИМИ
З ХРОНІЧНИМ ЗАПАЛЕННЯМ
Т. В. Мелешко , О. В. Паллаг
Р. О. Рукавчук , Л. С. Юсько , Н. В. Бойко
Ужгородський національний університет,
кафедра клініко-лабораторної діагностики
та фармакології, стоматологічний факультет,
Україна
Ужгородський національний університет,
науково-дослідний і навчальний центр
молекулярної мікробіології та імунології
слизових оболонок, Україна
E-mail: meleshkotv@ukr.net
Метою роботи було дослідити кишкову
мікробіоту у пацієнтів з некомунікативними
захворюваннями, пов’язаними з хронічним
запаленням, зокрема ожирінням, цукровим
діабетом 2-го типу, атеросклерозом та сер-
цево-судинними захворюваннями, а також
з’ясувати потенційну здатність екстрактів
плодів їстівних рослин пригнічувати ріст ок-
ремих умовно-патогенних мікроорганізмів.
В обмеженому клінічному дослідженні
аналіз мікробіоти кишечника проводили ру-
тинним методом, а також за допомогою qPCR.
Вивчено антибактеріальні властивості екстрак-
тів плодів їстівних рослин стосовно відібраних
умовно-патогенних мікроорганізмів.
Склад кишкової мікробіоти пацієнтів з
ожирінням характеризувався збільшенням
кількості Enterococcus spp. та Lactobacillus
spp. поряд зі зменшенням кількості
Escherichia coli. Зниження рівня E. coli та лактобактерій спостерігали у пацієнтів з цук-
ровим діабетом 2-го типу. За атеросклерозу
відзначали збільшення стрептококів, ентеро-
коків та ентеробактерій, тоді як у пацієнтів
із серцево-судинними захворюваннями на-
явним було додаткове підвищення кількості
стафілококів та кандид поряд зі зниженням
E. coli. Зменшення кількості Bifidobacterium
spp., Bacteroides spp., Roseburia intestinalis
та Аkkermansia muciniphila спостерігали у
пацієнтів усіх груп. Ріст Klebsiella spp. при-
гнічували екстракти червоної смородини і
сливи; Enterobacter spp. — екстракт череш-
ні; Proteus spp. — екстракти чорниці та ки-
зилу; Staphylococcus spp. — екстракти чорної
смородини, черешні, сливи, йошти, аличі та
кизилу.
Отримані дані можуть бути використані
для ранньої діагностики некомунікативних
захворювань та для їхньої профілактики за
допомогою персоніфікованого харчування.
T. V. Meleshko1, 2 1 Uzhhorod National University, Department
O. V. Pallah1, 2 of Clinical Laboratory Diagnostics and Pharmacology,
R. O. Rukavchuk2 Faculty of Dentistry, Ukraine
L. S. Yusko1, 2 2 Uzhhorod National University,
N. V. Boyko1, 2 Research Development and Educational Centre
of Molecular Microbiology and Mucosal Immunology, Ukraine
E-mail: meleshkotv@ukr.net
Received 27.07.2020
Revised 08.10.2020
Accepted 31.10.2020
Підписуйтесь на наші соціальні мережі, щоб стежити за останніми новинами тут 💜:
Сайт: www.ediens.me
LinkedIn: www.linkedin.com/ediens
Instagram: www.instagram.com/ediens_official
TikTok: www.tiktok.com/@ediens_official



Коментарі